The Color of the Future
A history of blue 🦋
My favorite color has changed throughout my life, cycling through the entire spectrum of visible light and beyond. I don’t remember when blue was the chosen one, exactly; maybe when I was 13 or so. After that, yellow, purple, orange, green, and pink occupied the top spot for various periods. Blue never made a comeback. I saw it as a banal, common color. After all, the sky is made of it, and the sky is everywhere.
Then I realized when compiling the tech tree that blue is the most fascinating color, because it is the hardest of the common colors to create artificially.1 You can’t just take a piece of the sky and put it into a painting. And blue pigments are fairly rare in minerals, plants, and animals. So blue had to be invented, time and time again, from 4000 BC to the 21st century. It is the most technological color, and I’m willing to claim that this is why it is usually, in science fiction and elsewhere, used to represent the future.
The story of blue starts with indigo. It is an organic dye made from plants in the Indigofera genus, which grow throughout the tropical and subtropical regions of the world. The first known traces of indigo dye come from the New World, in ancient Peru, 6,000 years ago, using Indigofera suffruticosa, or anil.2 In the Old World, it was known from Africa to East Asia, but became particularly associated with India (hence indi-go), where Indigofera tinctoria was domesticated. Indigo soon became a luxury, traded from India to Greco-Roman and then medieval Europe, where the same blue dye could only be made from a less productive plant, woad or Isatis tinctoria. Eventually the “blue gold” became an important colonial crop in the Caribbean and was part of the story of slavery, next to sugar, tobacco, and cotton.

Before indigo was a thing in the Old World (that started circa 2400 BC), the Egyptians had already become obsessed with the color blue. Besides the sky, it was available in the form of semiprecious stones like turquoise and lapis lazuli, cobalt oxide (more on that later), as well as the mineral azurite, which they mined in Sinai and the Eastern Desert.

Azurite would later enjoy a fruitful career as the main blue pigment in European painting, but to the Egyptians it was costly, and besides it isn’t the most stable blue color: it degrades and fades when in contact with air. And so they created the first synthetic pigment in history: Egyptian blue. The oldest evidence of it is in a bowl dated to 3250 BC. Egyptian blue is a calcium copper silicate with formula CaCuSi4O10 or CaOCuO(SiO2)4. Its method of manufacturing, in a rare example of lost technology, was forgotten towards the end of antiquity, but has been plausibly reconstructed. It likely involved heating together quartz sand (silica) and some source of copper (either copper ores or scraps from the bronze industry), together with an alkali (like natron) and a calcium oxide (unintentionally added as impurities in the other materials).

In another cradle of civilization, a very similar story unfolded from about 800 BC. So similar, in fact, that it has been speculated that knowledge of Egyptian blue spread along the early silk road, all the way to China, where Han blue (together with Han purple) makes an appearance during the Zhou dynasty. Han blue has almost the same chemical formula as Egyptian blue, but replaces calcium with barium: BaCuSi4O10. It may also have been an independent invention, perhaps the work of Taoist alchemists and glassmakers. Its use declined after the Han dynasty, and few examples survive.

Much later, China would become famous for another application of blue: the “blue and white” porcelain style. The blue here comes from cobalt oxide, which had colored Egyptian faience since at least 1500 BC, though nobody at the time knew what cobalt was. You could make cobalt blue in the form of glass and then grind it into a pigment called smalt. Despite porcelain originating in China, it seems that the use of smalt for the blue and white style began in Iraq. It spread from the Middle East to China, and then from China to the rest of the world including Europe, where it would eventually allow Swedish chemist Georg Brandt to identify cobalt as an element in 1735, the first time a new metal was discovered since antiquity.3

Meanwhile, in the New World, the local indigo dye was being combined with a clay called palygorskite to create what became known as Maya blue, which was the main blue pigment in Mesoamerican art from about 800, and was still used as late as the 19th century, though it, too, was forgotten about for a while.

But none of the pigments mentioned so far, not azurite, not cobalt blue, not Egyptian blue, could rival with the purest and deepest of blues, the one that came from grinding the rare lapis lazuli stone into a powder. Lapis lazuli had been extracted from mines in what is now Afghanistan since ancient times, but was used for paint starting around the 5th to 7th centuries, for use in Zoroastrian and Buddhist religious art. This pigment became known to medieval and Renaissance Europeans as ultramarine, meaning “beyond the sea,” since it had to be imported at great cost from central Asia (which, to the Venetian merchants who mostly controlled this trade, was beyond the Mediterranean sea, I suppose). Nobody has written about this more eloquently than Scott Alexander:
Here is the process for getting ultramarine. First, go to Afghanistan. Keep in mind, you start in England or France or wherever. Afghanistan is four thousand miles away. Your path takes you through tall mountains, burning deserts, and several dozen Muslim countries that are still pissed about the whole Crusades thing. Still alive? Climb 7,000 feet through the mountains of Kuran Wa Munjan until you reach the mines of Sar-i-Sang. There, in a freezing desert, the wretched of the earth work themselves to an early grave breaking apart the rocks of Badakhshan to mine a few hundred kilograms per year of blue stone - the only lapis lazuli production in the known world.
Buy the stone and retrace your path through the burning deserts and vengeful Muslims until you’re back in England or France or wherever. Still alive? That was the easy part. Now you need to go through a chemical extraction process that makes the Philosopher's Stone look like freshman chem lab. “The lengthy process of pulverization, sifting, and washing to produce ultramarine makes the natural pigment … roughly ten times more expensive than the stone it came from.”
Finally you have ultramarine! How much? I can’t find good numbers, but Claude estimates that the ultramarine production of all of medieval Europe was around the order of 30 kg per year - not enough to paint a medium-sized wall. Ultramarine had to be saved for ultra-high-value applications.
In practice, the medievals converged on a single use case - painting the Virgin Mary’s coat.
By the beginning of the 18th century, Egyptian blue had been long forgotten, and painters in Europe relied on indigo, smalt, azurite, and when they could get their hands on it, ultramarine. But this was the modern, enlightened era of European science. Great things were to come.
It began with a chance discovery. In Berlin around 1706, a paintmaker, Johann Jacob Diesbach, was trying to prepare red dye from cochineal.4 The details of the story are not totally ascertained, but it seems that his intended mix of cochineal insects, ferric sulfate, and potash had been tainted by another substance, perhaps bone oil from the alchemist Johann Konrad Dippel. The result was a deep blue pigment, soon to be known as Prussian blue, since Berlin was the capital of Prussia. It immediately found its niche in the art market: a deep blue, like ultramarine, but which unlike ultramarine didn’t cost more than literal gold. Within a couple of years, painters were already depicting the Virgin Mary’s robes with Prussian blue.
Thus Prussian blue became the first modern synthetic pigment. It spread far and wide, even to isolationist Japan. Large quantities of Prussian blue began entering the country around 1829, through the single trading post the Japanese allowed with Westerners, at Dejima, and very soon after, revolutionized the woodblock printing art of ukiyo-e. As early as 1831, one of the most famous works in art history was created with abundant Prussian blue.
Prussian blue is also the blue of blueprints, created with the cyanotype process, one of the first ways to make many copies of a document. The blueprint was invented in 1842 by John Herschel and became the standard for engineering drawings; it was also used abundantly to duplicate photographs. Though it has become obsolete (replaced by whiteprint and then xerography, the currently dominant photocopying technique), it survives as the word to describe any technical, detailed plan.
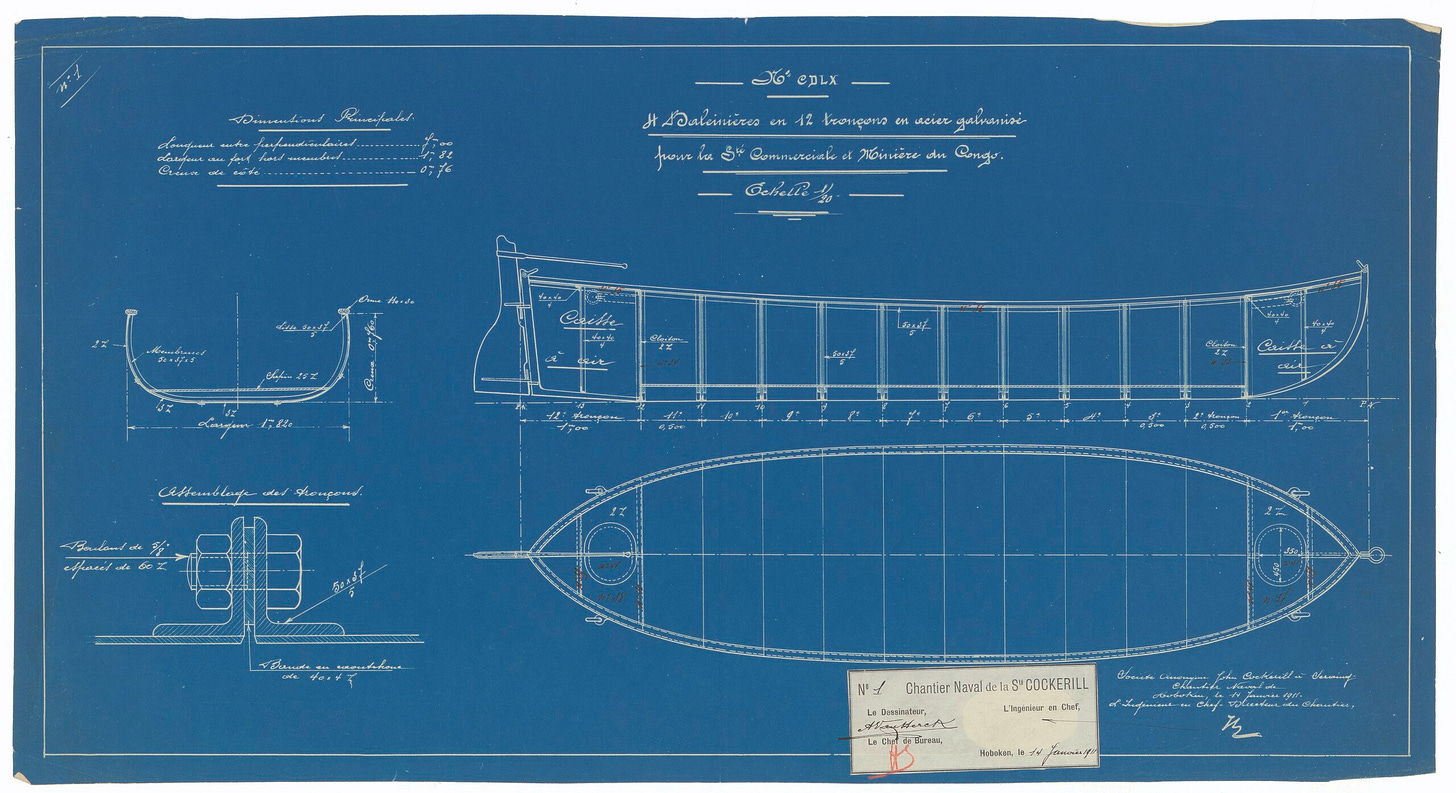
Prussian blue was only the first of a series of synthetic blue pigments that span the history of industrial civilization. In 1789, cerulean appears, the creation of Albrecht Höpfner in Switzerland. It is another compound of cobalt, but combined with tin: a cobalt stannate (Co2SnO4). It would become available to artists in paint form only in the middle of the 19th century.
Around the same period, in 1799 or 1802 (sources differ), the French chemist Louis Jacques Thénard reinvented cobalt blue. It was a commission from another chemist, Jean-Antoine Chaptal, who happened to be a minister in the government of the First French Republic. Thénard investigated the pigments at the Sèvres porcelain factory, but used a different method than the originators of smalt pigments in Egypt, Iraq, or China, using aluminium (formula: CoAl2O4). By the middle of the 19th century, the leader in the production of cobalt aluminate was Blaafarveværket, a large industrial enterprise in Norway.
In this golden age of blue pigment synthesis, would it be possible to create even synthetic ultramarine? Goethe, already, had noticed the blue deposits on lime kilns when visiting Sicily in 1787. The locals used it for decoration as if it were lapis lazuli. The same phenomenon was observed in limeworks in France in the 1810s, and in 1824, the Société d’encouragement pour l’industrie nationale — a government organization dedicated to further French industry in response to the industrial revolution in Britain, and led by the aforementioned Jean-Antoine Chaptal — announced a prize of 6,000 francs to anyone who could make ultramarine for much cheaper than the price of lapis lazuli. In 1826, Jean-Baptiste Guimet succeeded in Lyon. He won the prize and established a thriving business, though he kept his methods secret and, as a result, forever has to share credit with Christian Gmelin in Tübingen, who did publish the process. It involves heating up clay, sodium hydroxide, and coal together.5

Artists and decorators now had their main blue pigments. Soon, industry and science would extend the use of blue to other domains. In 1897, it became practical to prepare artificial indigo in industrial quantities, eventually replacing all use of the plant. Today 80,000 tonnes are produced per year, mostly for the purpose of dying textiles and especially the denim of jeans.
Around the turn of the 20th century, artificial food colorings became widespread, derived primarily from coal tar. One of them would become known as brilliant blue FCF or Blue No. 1. Together with Blue No. 2, which is made from indigo, it is one of the two main blue colorings, and has a strong association with the familiar-yet-mysterious blue raspberry flavor.
The 1920s saw the introduction of another synthetic pigment, phthalo blue (also known as copper phthalocyanine), perhaps in a way harking back to the copper-derived compounds of ancient Egypt. It has grown to be most widely produced blue.

Though the discovery of new pigments is a rare occurrence, it still happens. In 2009, a serendipitous discovery at the Oregon State University led to YInMn Blue, so named because it contains yttrium, indium, and manganese, and pronounced “yinmin.” It is a near-perfect blue that furthermore avoids the toxicity and environmental problems of the pre-existing pigments.
I have this half-baked theory that science fiction is associated with blue because of blue LEDs.
Consider this chart, which I wrote about in an old post:
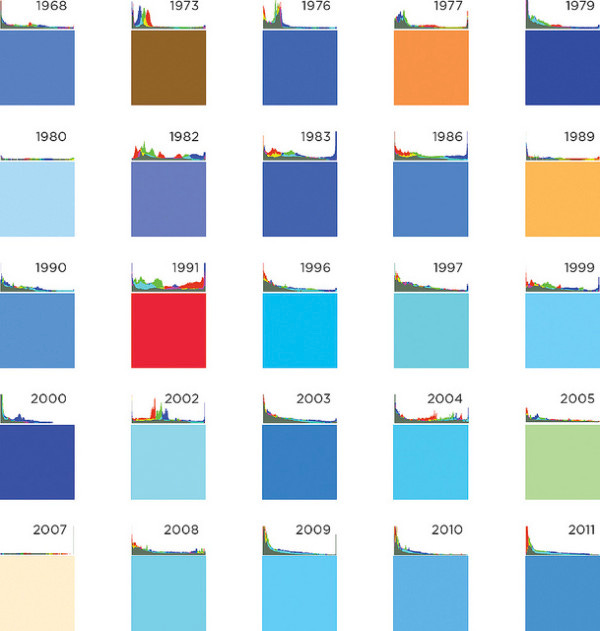

There are a number of competing hypotheses for why science fiction movie directors and video game designers overwhelmingly choose blue as the color of fictional user interfaces. They include:
Accidental reasons from filmmaking considerations (from Mark Coleran):
Using simple interfaces with primary RGB colors on black looks better in film than ordinary liquid-crystal screens, so most UIs in video media is either red, green, or blue
Red is associated with weapons, and green with vintage electronics (which commonly used green-phosphor monochrome monitors), leaving blue as the generic and/or futuristic choice
Blue is easier to color-correct: a lot of filmed material tends to look blue before color correction, but you don’t need this when the image is supposed to be blue
Cultural associations:
Blue fits well with science fiction thanks to associations like coldness, knowledge, otherworldliness, and creative transcendence (found in some academic paper in Korean thanks to Elicit)
Something something near-far Robin Hanson something something6
Blue is rare in nature except the for sea and sky, so “there’s something fundamentally mystical, unnatural, and inhuman about it” (from Noessel, cited in ‘Future Screens are Mostly Blue’)
Most science fiction creators simply copy the tropes of existing science fiction, so they choose blue because it already is the “science fiction color.” (And picking something else is likely to be interpreted as an intentional deviation for a specific purpose.)
I’d guess that the actual, immediate reason for most blue in science fiction is the last one. Unless creators make a conscious artistic choice to deviate, they tend to copy what’s typical and expected in their chosen genre. Yet those norms and expectations have to come from somewhere. It’s possible that the items in the first part of the list, about decisions having to do with the techniques of filmmaking rather than with the artistic meaning of blue, are the actual cause for some early shows that snowballed into the ubiquity of blue interfaces today, but that seems a bit like post-hoc justification to me unless we can find evidence of those decisions being made.
So it’s probably cultural associations, but most of them also just kick the can further. Overall I suppose I somewhat agree with Noessel: it may well come down to the difficulty in finding blue in nature. Or finding it in technology, considering the history of blue pigments that we just went over.
And not just pigments. There is another realm in which blue has proven incredibly difficult to produce: light. In fact we found the solution so recently that it is why, I speculate, the future is still strongly associated with blue.
The story of how we produce light is a fun one, spanning all of our technological history and involving dozens of solutions, from prehistoric oil lamps to candles to coal gas to cold-cathode tubes. But most of those solutions have produced light somewhere between white and the reddish yellow of flames or black-body radiation. If you wanted blue light, you could make a bulb out of blue glass (with cobalt!) and put an incandescent filament in it. This worked okay, but blue light bulbs did tend to be less satisfying than the other colors, or at least that’s what I remember from Christmas lights when I was a kid.7
There were other strategies for blue lights: construct a tube like the familiar red-glowing neon ones, and put mercury vapor in it. Low-brightness phosphors for RGB screens. Greenish-blue vacuum fluorescent displays. So, blue light was not an unsolved problem. But it wasn’t as conclusively solved as light in the other spectral colors.
In the 1960s, light-emitting diodes started becoming practical. LEDs are the most efficient way of creating light by far, but the properties of the materials they are made of — semiconductors that emit light when traversed with an electrical current — make it much easier to generate radiation in the less energetic part of the electromagnetic spectrum. Thus the first practical LED emitted infrared light, by Texas Instruments in 1962. Later that same year, the first visible-light LED was made at General Electric, in red. Displays made of red LEDs soon became widespread in electronic devices (replacing Nixie tubes, also reddish) after some further advances by Hewlett-Packard circa 1968.

Humanity then gradually conquered the rest of the visible light spectrum, with orange, yellow, and green LEDs being developed in the 1970s. But blue remained elusive. A practical, bright blue LED would not be made, despite much research being poured into it by electronics companies around the world, until a breakthrough by Shuji Nakamura in Japan in 1993.
This completed the color spectrum and enabled us to create white LEDs, which are now quickly replacing nearly every lighting technology since they cost so little and are so customizable. We can say we have essentially “solved” lighting. Blue LEDs also enabled the first blue lasers in the mid-1990s.
This is a very recent development! For a very long time, blue would have been the color that only “future tech” could create. Then, for a brief period, it would have been the color of cutting-edge tech. Now, 30 years later, the tech exists and is widespread, but we still have the memory of that time. And furthermore no other color can take its place as the inaccessible one; we’ve conquered the entire spectrum.8

Does the futuristic quality of blue really come from LEDs? Maybe, maybe not. I’m not sure there’s a direct causal link.
But given the full history of blue pigments, I wouldn’t be surprised to find some truth in this speculative scenario: that there were just enough innovations in blue, a steady trickle of serendipitous discoveries and long-term research efforts to produce better versions of it, to keep it in the mind of humans as the color of the artificial and the cutting-edge. If you wore indigo-dyed clothes in ancient India, you were one step more removed from nature than the person who wore plain cotton. If you used blue pottery in Egypt or Iraq or China, you were clearly cooler than the people who used plain terracotta. If you hired an engineer or architect at the peak of the Industrial Revolution in the late 19th century, they’d be way more efficient at their job if they duplicated their drawings with Prussian blue instead of copying them by hand. And today, if you want to make your city a herald of the high-tech future, you decorate everything with programmable blue LEDs. No other color would do.
This post was written as part of the Roots of Progress Institute’s Blog-Building Intensive, and I thank the fellows who provided feedback: Allison Lehman, Kelly Vedi.
One could say the same about purple, which has its own history of being a super expensive pigment, Tyrian purple, and holds the distinction of being one of the first synthetic dyes, mauveine. But purple is less common and important than blue. Besides, it doesn’t really exist.
While we’re here, let’s note that Tyrian purple, made from sea snails, may be related to a blue dye of great significance in Jewish culture, tekhelet. It has been speculated that tekhelet comes from Hexaplex trunculus snails. I didn’t mention it in the main text because its origin is uncertain.
From which we derive the word aniline, a common industrial chemical that is nowadays used to make indigo and various other dyes.
Fun fact: cobalt is named after kobolds, mischievous spirits from German folklore, because miners in Germany would attribute to them the unusual properties of the ore containing the metal.
As an aside, the history of cochineal dye, made from insects grown on cactus according to secret ancestral techniques of the Zapotec people, and the second-highest valued export from New Spain after silver, is fascinating in its own right. By the way it’s still used as food coloring, so if you eat artificially red foods, you probably eat insects.
Synthetic ultramarine is also (in)famous for being the main component (together with a resin) of International Klein Blue, the creation of artist Yves Klein, who painted large monochrome paintings with it.
Specifically posts like ‘Is Blue Far?’ and ‘Near Far in Science Fiction’. Blue might be associated with “far” in terms of construal level theory and likewise for science fiction, which makes an association natural. I thought this was a mind blowing point when I first encountered it some years ago but now it seems rather unconvincing, which is why I’m relegating it to a footnote.
I think it’s because the filaments glow yellow, and a lot of the light is filtered by the blue glass, leaving dim light bulbs. But also the blue glass tended to become discolored, and then you’d just get a plain white bulb.
There can’t be a purple LED since purple isn’t a spectral color, with the exception of violet. And violet LEDs appeared about the same time as the blue ones. There is active development of ultraviolet LED, especially for disinfecting lamps, but of course we won’t be able to see them.
I suppose one intriguing possibility would be if we were to invent new colors altogether, by modifying the biology of color perception. Then maybe the color of the future would become octarine or something.





![Sassoferrato's depiction of the Blessed Virgin Mary, The Virgin in Prayer, c. 1654. Her blue cloak is painted in ultramarine.[33] Sassoferrato's depiction of the Blessed Virgin Mary, The Virgin in Prayer, c. 1654. Her blue cloak is painted in ultramarine.[33]](https://substackcdn.com/image/fetch/$s_!QzTl!,w_1456,c_limit,f_auto,q_auto:good,fl_progressive:steep/https%3A%2F%2Fsubstack-post-media.s3.amazonaws.com%2Fpublic%2Fimages%2F494aee97-8608-4c9a-947f-bc9a609a1e8c_1920x2415.jpeg)





Hmm, and the emerging Prussian Army under the Hohenzollerns adopted blue uniform coats.
I think the science fiction angle comes from the fact that engineers use blueprints. If you look at the sci-fi screen shots in the article, they look similar to modern blueprints.